Background: Langerhans cell histiocytosis (LCH) is an inflammatory myeloid neoplasia characterized by lesions including pathogenic CD207+ dendritic cells among an inflammatory infiltrate with recurrent, mutually exclusive somatic activating mutations in MAPK pathway genes in ~85% of LCH lesions. Our group and others have reported that Hispanic populations are more likely to develop LCH, particularly high-risk LCH, than non-Hispanic counterparts. Our group previously identified and validated a risk SNP in SMAD6 associated with a 3-fold increase in LCH risk that was also enriched in those of Hispanic ancestry; however, germline risk factors have yet to be fully elucidated in the higher-risk Hispanic population.
Methods: Using targeted SMAD6 sequence data from 466 cases with LCH and aggregate data for 15,694 non-cancer controls from gnomAD v2.1.1, we evaluated 49 SNPs previously associated with case status to identify those that were enriched specifically in Hispanic populations. We conducted an additional genome-wide association study (GWAS) on 98 LCH cases and 1,158 controls, all inferred to be Hispanic based on global genomic ancestry. The GWAS was adjusted for the first 3 PCs. Population genetics were assessed in Ensembl, ancestry inference was conducted Admixture software (reference panel 1000 Genomes, Phase 3), and statistical analyses were conducted with PLINK, Stata, and SAS software.
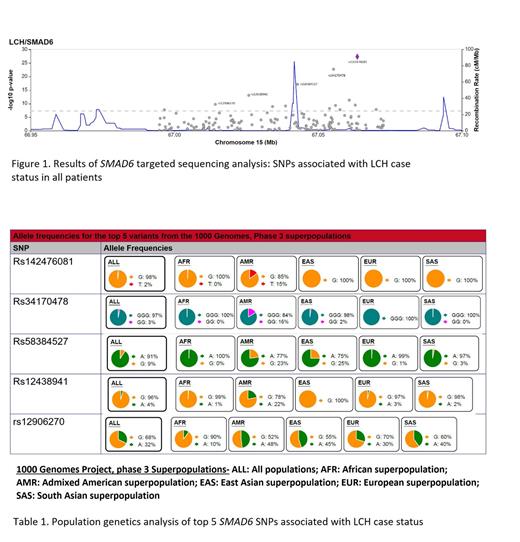
Results: In the population genetics analysis of the SMAD6 SNPs, the top 4 SNPs (Figure 1) have a lower minor allele frequency (MAF) of 2-9% across all populations. However, in Hispanic populations, the alternate/risk allele in the top 4 SNPs is increased (MAF range 15-23%). Furthermore, the LD between the top four SNPs is enriched among Hispanic populations (range of r 2: 0.51-0.92; Table 1), suggesting that Hispanic patients may have different risk loci compared to others. In the LCH GWAS of Hispanic patients, there were no statistically significant SNPs; however, this pilot study of our ongoing analyses identified 5 LCH risk loci in Hispanic patients that had suggestive significant associations ( P=1.0x10 -5). Strikingly, these loci differed from the SNPs identified in our GWAS of all patients.
Conclusions: Using multiple approaches, we have identified LCH risk variants that were identified in Hispanic patients. These Hispanic ancestry-specific risk SNPs were more strongly associated with case status in Hispanic patients than the risk SNPs from our GWAS of all patients. This indicates that the ancestry-specific SNPs may contribute to the increased risk of LCH, particularly high-risk LCH, that is seen in Hispanic populations compared to populations of other ancestries. Our ongoing work involves assessing these SNPs for association with patient and clinical characteristics and outcomes in Hispanic patients. In addition, we are determining how these Hispanic ancestry-specific variants affect risk of LCH in patients of other ancestries, identifying additional ancestry-specific risk SNPs in patients of other ancestries, and examining the effects of global and ancestry-specific risk SNPs on LCH risk.
Disclosures
Allen:Sobi, Inc: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal